In Vitro Diagnostic Device Labeling Requirements . In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices what is the ivdr? The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. what are the requirements for ivd labeling? the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices.
from www.tuvsud.cn
the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. what are the requirements for ivd labeling? this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices. In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. what is the ivdr? The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide.
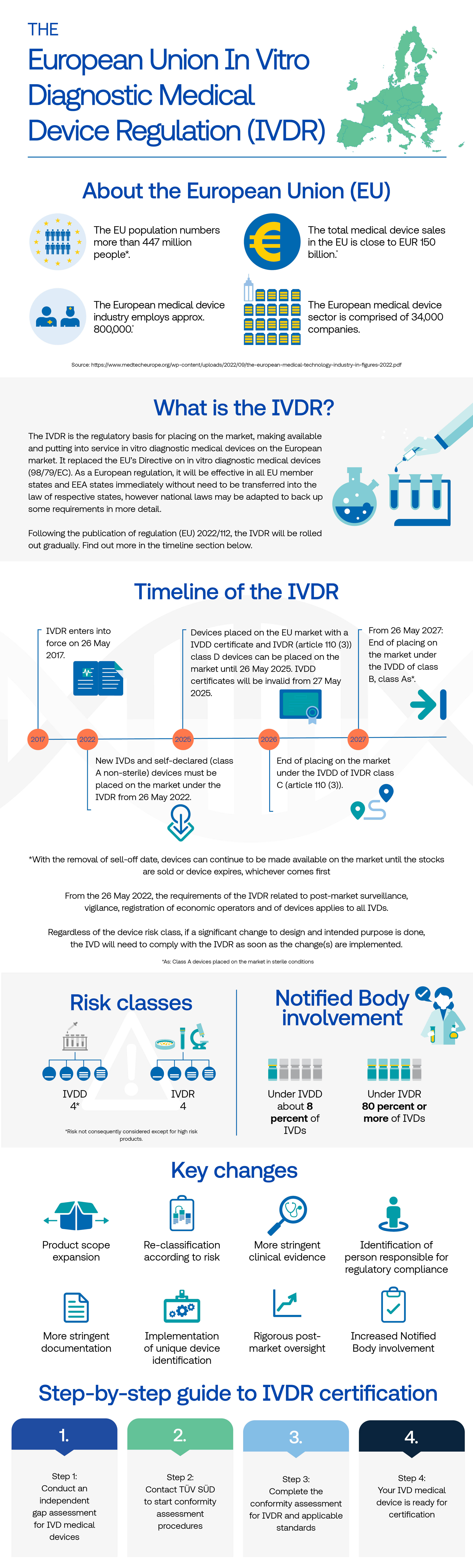
Infographic The In Vitro Diagnostic Medical Device Regulation TÜV南德
In Vitro Diagnostic Device Labeling Requirements In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. what is the ivdr? what are the requirements for ivd labeling? guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices.
From ddregpharma.wixsite.com
Regulatory Requirements for InVitro Diagnostic Devices In Vitro Diagnostic Device Labeling Requirements The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices what are the requirements for ivd labeling? what is the ivdr? The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will. In Vitro Diagnostic Device Labeling Requirements.
From standards.iteh.ai
EN 3761992 In vitro diagnostic systems Requirements for labelling In Vitro Diagnostic Device Labeling Requirements guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. what are the requirements for ivd labeling? the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. The objective of the asian. In Vitro Diagnostic Device Labeling Requirements.
From www.qvccert.com
InVitro Diagnostic Medical Devices CE Marking CE Marking, Ce Mark In Vitro Diagnostic Device Labeling Requirements this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices. the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. what is the ivdr? what are the requirements for ivd labeling? In vitro diagnostic products have additional labeling requirements under 21 cfr. In Vitro Diagnostic Device Labeling Requirements.
From www.tuvsud.cn
Infographic The In Vitro Diagnostic Medical Device Regulation TÜV南德 In Vitro Diagnostic Device Labeling Requirements what is the ivdr? In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices the purpose of this imdrf guidance is to provide globally. In Vitro Diagnostic Device Labeling Requirements.
From www.regdesk.co
FDA Guidance on Labeling for In Vitro Diagnostic Devices RegDesk In Vitro Diagnostic Device Labeling Requirements guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. what are the requirements for ivd labeling? The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. the. In Vitro Diagnostic Device Labeling Requirements.
From www.thermofisher.cn
IVDD vs. IVDR Classifications Defined and Compared OEMpowered In Vitro Diagnostic Device Labeling Requirements In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. what is the ivdr? what are the requirements for ivd labeling? this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices. The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. the. In Vitro Diagnostic Device Labeling Requirements.
From standards.iteh.ai
ISO 1811312022 In vitro diagnostic medical devices — Information In Vitro Diagnostic Device Labeling Requirements guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices what is the ivdr? The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. the purpose of this. In Vitro Diagnostic Device Labeling Requirements.
From www.bsigroup.com
In Vitro Diagnostic Regulation IVDR Medical Devices BSI America In Vitro Diagnostic Device Labeling Requirements what are the requirements for ivd labeling? what is the ivdr? The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will. In Vitro Diagnostic Device Labeling Requirements.
From ceqcidjn.blob.core.windows.net
Eu Mdr Medical Device Labeling Requirements at Mary Plank blog In Vitro Diagnostic Device Labeling Requirements what is the ivdr? In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices what are the requirements for ivd labeling? The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. this guidance. In Vitro Diagnostic Device Labeling Requirements.
From mdrc-consulting.com
Europe's IVD regulatory approval process MDRC In Vitro Diagnostic Device Labeling Requirements The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. what are the requirements for ivd labeling? this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices. The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. . In Vitro Diagnostic Device Labeling Requirements.
From www.regdesk.co
FDA Guidance on Labeling for In Vitro Diagnostic Devices RegDesk In Vitro Diagnostic Device Labeling Requirements what are the requirements for ivd labeling? The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices.. In Vitro Diagnostic Device Labeling Requirements.
From www.dentalhealth.vip
Deciphering In Vitro Diagnostics (IVD) Medical System Rules DentalHealth In Vitro Diagnostic Device Labeling Requirements what is the ivdr? the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. what are the requirements for ivd labeling? The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. this guidance document is intended to assist manufacturers in. In Vitro Diagnostic Device Labeling Requirements.
From mdssar.com
MDR and IVDR Services The MDSS Solution! In Vitro Diagnostic Device Labeling Requirements The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. what are the requirements for ivd labeling? guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. this. In Vitro Diagnostic Device Labeling Requirements.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide In Vitro Diagnostic Device Labeling Requirements guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. what is the ivdr? what are the requirements for ivd labeling? In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. The. In Vitro Diagnostic Device Labeling Requirements.
From www.tuvsud.cn
Infographic The In Vitro Diagnostic Medical Device Regulation TÜV南德 In Vitro Diagnostic Device Labeling Requirements The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices. The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. In vitro diagnostic products have additional labeling requirements under. In Vitro Diagnostic Device Labeling Requirements.
From www.regdesk.co
FDA Guidance on Labeling for In Vitro Diagnostic Devices RegDesk In Vitro Diagnostic Device Labeling Requirements guidance for additional considerations to support conformity assessment of in vitro companion diagnostic medical devices what are the requirements for ivd labeling? this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices. The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. In vitro diagnostic. In Vitro Diagnostic Device Labeling Requirements.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk In Vitro Diagnostic Device Labeling Requirements this guidance document is intended to assist manufacturers in the labelling of in vitro diagnostic devices. the purpose of this imdrf guidance is to provide globally harmonized labeling principles for medical devices, including in. what is the ivdr? The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. . In Vitro Diagnostic Device Labeling Requirements.
From www.en-standard.eu
BS ISO 211512020 In vitro diagnostic medical devices. Requirements for In Vitro Diagnostic Device Labeling Requirements The objective of the asian harmonization working party (ahwp) is to encourage convergence at the worldwide. The in vitro medical devices regulation (eu) 2017/746 (ivdr) is a new regulation that will create a robust,. In vitro diagnostic products have additional labeling requirements under 21 cfr 809,. guidance for additional considerations to support conformity assessment of in vitro companion diagnostic. In Vitro Diagnostic Device Labeling Requirements.